Every cell, tissue, organ, gland, and system in animals requires certain trace minerals in order to function normally. Trace minerals such as zinc, copper, and manganese, absorbed mostly from the diet, are essential for numerous biological processes. Deficiencies can result from poor dietary absorption and cause a host of problems, ranging from poor growth performance to disease.
Formulated diets may include inorganic salts as sources of zinc, copper, and manganese, but can result in poor bioavailability of the minerals due to numerous chemical antagonisms and interactions with other components in the diet. Practicing nutritionists can achieve better bioavailability of trace minerals through organic sources in which the minerals are chemically bound to an amino acid, organic acid, protein, or carbohydrate moiety, called a ligand.
Zinc is the most common metal constituent of cellular enzymes, and as such plays essential roles in cell proliferation and death, immune development and response, reproduction, gene regulation, and defense against oxidative stress and damage. Two key structural proteinscollagen and keratinboth require zinc for their synthesis. The importance of these two proteins is clear. Keratin is the major structural protein of hooves, feathers, skin, beaks, and claws, while collagen is the major structural protein of extracellular matrix and connective tissues in internal tissues, including cartilage and bone.
Like zinc, copper is essential for a wide variety of health and performance-related functions in all animal species. Copper is essential for reproduction and embryo development. Copper-dependent enzymes enhance metabolic processes that require zinc. For example, lysyl oxidasea copper-dependent, cross-linking enzyme, increases the strength of collagen and elastin. Because of its role in cross-linking, copper enhances the strength of skin, bone, intestines, and arteries, including the aorta.
A third trace mineral, manganese, plays an important role in bone development, both in the embryo and after birth. Manganese is required for multiple steps in the synthesis of the proteoglycan matrix of developing bone. Proper development of this matrix is required for later stages of bone development and calcification. Like zinc and copper, manganese also participates in the antioxidant defense of cells, as a cofactor in the manganese-dependent form of the protective enzyme superoxide dismutase.
Deficiencies in these and other trace minerals cause a host of diseases and metabolic disorders which can affect food animals (see table). In commercial livestock and poultry production, trace mineral deficiencies can result in poor growth and feed efficiency, and a higher incidence of disease despite vaccination. In meat production, signs of a trace mineral deficiency include carcass bruising and broken bones, which reduce value of saleable product.
Traditional trace mineral sources
Traditionally, livestock and poultry nutritionists have supplemented feed with inorganic salts of zinc, copper, and manganesemainly in the form of oxides and sulfates. However, these inorganic salts can result in poor bioavailability of the mineral, primarily due the numerous antagonisms and interactions between the inorganic salts and other components in the digesta. An example of a dietary antagonist for trace minerals is phytate, the predominant form of phosphorus in grain and other plant material. Phytate binds trace minerals, creating insoluble and thus unavailable compounds that are excreted from the animal's digestive tract in the feces. Antagonisms can also occur between one trace mineral and another. For example, high levels of zinc reduce the availability of copper.
.jpg?auto=format%2Ccompress&fit=max&q=70&w=400) 0609FMtrace
0609FMtrace
The common denominator in these antagonisms is the dissociation of the mineral cation from its corresponding anionoxide or sulfatein the relatively high-acid environment of the anterior gastrointestinal system. Acidic conditions occur in the stomach in pigs, the omasum in ruminants, and the crop, proventriculus, and gizzard in poultry species. When the dissociated trace mineral reaches the more neutral pH environment of the small intestine, it binds to the components of the digestasuch as phytaterendering the trace mineral insoluble and unavailable for absorption.
Trace mineral antagonisms often result in metabolic deficiencies in livestock and poultry. However, it is possible to avoid the problem of antagonisms by replacing all or part of the inorganic trace minerals in premix products with organic trace minerals (OTMs). These OTMs consist of trace minerals chemically bound to an amino acid, organic acid, protein, or carbohydrate moiety, called a ligand.
The ligand link
Organic trace minerals improve the trace mineral status in livestock and poultry through the ‘complexation' or chelation of the ligand to the trace mineral atom. This type of bonding creates a stable complex in the upper portion of the gastrointestinal system, where acidic, low-pH conditions prevail. The resulting enhanced stability of the complex or chelate reduces dissociation of the mineral in the upper gastrointestinal tract and therefore minimizes trace mineral losses due to antagonisms. The complex or chelate can thus deliver the mineral in a protected form to the absorptive epithelium of the small intestine for uptake by the body. The organic component of the organic trace mineral functions as the link or ‘delivery vehicle'. The metabolic benefits of organic trace minerals are the result of increased mineral delivery to the small intestine, rather than as a direct function of the organic trace mineral complexes themselves.
As organic forms of zinc, copper, and manganese have come into greater use, researchers in a number of laboratories have compared the bioavailability of inorganic and organic trace minerals in several species and classes of animals. Most researchers have agreed that the absorption rate of minerals from OTMs can be substantially greater than those from inorganic forms. However, researchers have found that organic trace minerals are not equally stable at low pH, and therefore some have a limited ability to increase the bioavailability of a given trace mineral.
Recently, an international feed ingredient manufacturer developed a new generation of organic trace minerals in which two molecules of 2-hydroxy-4 (methylthio) butanoic acid (HMTBA) are ‘complexed' or chelated to an atom of zinc, copper, or manganese to form a highly bioavailable source of these minerals. The structure of these chelates has been defined by a variety of assays, including X-ray crystallography (see figure Schematic'). When this molecule reaches the site of mineral absorption in the small intestine, the combined influence of the pH of the unstirred water layer of the intestinal mucosa and the strength of the mineral receptor on the intestinal cell membrane breaks the bonds of the molecule, freeing the trace mineral. The free trace mineral and the HMTBA are then absorbed separately across the epithelium for utilization in the animal.
Using a computer model, it is possible to compare the stability of zinc-HMTBA chelate (Zn[HMTBA]2) with another OTM, zinc-methionine, over a range of acidities typical in the digestive tract of the chicken (see figure Stability'). This graph predicts the stability of the zinc-HMTBA interaction versus the zinc-methionine interaction at a given concentration of zinc and ligand. At neutral pH, there is not a great difference in stability between zinc-HMTBA and zinc-methionine. However, as the pH decreasesconsistent with the pH levels found in the crop, proventriculus, and gizzardthe model predicts that zinc-HMTBA largely remains intact. In contrast, the zinc-methionine is predicted to dissociate. Such dissociated, unbound zinc is susceptible to antagonism or other forms of loss, reducing the availability of the mineral for absorption. It is important to note that the predictions of this model have been verified by bench-top experiments.
Following the absorption of the mineral, the two ligand HMTBA molecules are absorbed and utilized as a source of methionine activity. The trace mineral-HMTBA chelate has a much greater methionine activity than zinc-methionine80% versus 20% on a product basis. Furthermore, biochemical and growth performance experiments have demonstrated that the HMTBA from trace mineral-HMTBA chelates has the same methionine value as unchelated HMTBA. Therefore, in all feed formulations, the trace mineral-HMTBA chelate can replace a portion of the supplemental synthetic methionine.
Implications of optimum ligand stability
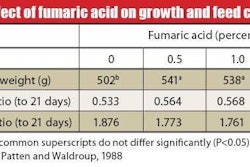
Researchers have recently completed several trials designed to measure the benefits of ligand-induced stability of trace minerals on animal performance. In one study, researchers compared three sources of zinc: Zinc-sulfate, zinc-methionine, and zinc-HMTBA in relation to the immune response in broiler chicks to a coccidiosis vaccine. Broilers supplemented with zinc-HMTBA produced numerically or significantly higher antibody titers against two coccidia antigensmicroneme protein and sporozoite surface antigenthan broilers supplemented with either zinc sulfate or zinc methionine (see figure Immune response').
Researchers also recently conducted a field trial with turkeys in the Midwest USA. Turkeys were fed diets with standard inorganic trace minerals, or diets supplemented w ith a blend of HMTBA-organic trace mineral chelates20 ppm zinc, 20 ppm manganese, and 10 ppm copperin addition to the inorganic minerals (see figure Leg weakness'). Turkeys fed the HMTBA-chelates exhibited significantly lower levels of tibial dyschondroplasia (TD). In addition, bone breaking strength was significantly higher in heavy turkeys, which had live body weight greater than 30 lbs (13.6 kg), supplemented with a blend of HMTBA-chelates. Furthermore, these birds also exhibited significantly lower incidence and severity of footpad lesions, consistent with the effects of trace minerals on foot tissue health.
Researchers also have reported a reduction in the turnover of intestine epithelial cells in broiler chicks when fed diets supplemented 40 ppm zinc from zinc-HMTBA. The researchers explained that a reduction in intestine cell turnover makes more metabolic energy available to the bird for growth, reproduction, and other functions. They pointed out that approximately 30% of the metabolizable energy content of broiler feed is utilized for intestine tissue function.
In other broiler studies, researchers supplemented diets adequate in copper or zinc with several commercial sources of inorganic and organic trace minerals. Ileum breaking strength was highest in the broilers supplemented with zinc-HMTBA or copper-HMTBA. A plausible explanation for the difference is that the HMTBA chelates allowed greater copper and zinc to be available for collagen cross-linking (in the case of Cu) and synthesis (in the case of Zn)two factors in tissue integrity.
As nutritionists reassess the forms of trace minerals they use in livestock and poultry feed formulations, a growing number are replacing all or some of the inorganic trace minerals with organic forms. However, not all organic trace minerals are equal in their availability to the animal. The stability of the complex or chelate in the acidic portion of the digestive tract has a major effect on the ability of the molecule to deliver the trace mineral to the site of absorption, and thus allow improved trace mineral availability. In its role as a ligand, HMTBA appears to have a substantial advantage over other ligands, such as methionine, proteinates, and carbohydrates, in terms of stability and maximizing the availability of trace minerals. Moreover, the HMTBA from HMTBA-trace mineral chelates has the same methionine value as unchelated HMTBA.