During the past five years, segments of the U.S. egg production industry have been impacted by elevated flock mortality due to peritonitis. In addition, many operations continue to experience erosive losses from airsacculitis due to secondary E. coli infection following exposure to Mycoplasma gallisepticum (MG) and viral respiratory pathogens including infectious bronchitis (IB), lentogenic Newcastle disease (ND) and laryngotracheitis (LT).
The common factor in primary and secondary infections is a range of disease-causing E. coli strains collectively termed Avian Pathogenic E. coli (APEC). Although E. coli is commonly found in poultry houses and is a normal component of intestinal flora, some strains have developed an increased capacity to cause disease. This is usually associated with genetic determinants located on a virulence plasmid.
Various strains of E. coli are associated with omphalitis which results in elevated chick mortality. A high prevalence of this condition usually denotes a deficiency in hygiene extending through the chain of production from breeder flocks to chick handling. Omphalitis is believed to be unrelated to the subsequent occurrence of either primary peritonitis or secondary airsacculitis.
Coliform bacteria are present in the feces of all flocks and become suspended in the airborne dust of poultry houses with both floor and caged-housed flocks.
Studies have documented over a million E. coli organisms per gram of dust especially in controlled-environment cage houses operated at low humidity levels which occur during winter in the Midwest. Contaminated water supplied to flocks is frequently implicated in APEC infection. Rodent droppings also may be heavily contaminated with E. coli and can be ingested from feed troughs.
Immunosuppression contributes to APEC
Immunosuppression is an important contributor to the prevalence and severity of APEC infection. Flocks which are exposed to immunosuppressive viruses during the early brooding period or are subjected to environmental stress or mycotoxins are unable to establish an effective cellular response resulting in failure to engulf and inactivate APEC at the point of entry in the respiratory, intestinal and reproductive tracts. The production of antibodies against APEC in immunosuppressed flocks is less efficient compared to flocks with an intact immune response.
This manifestation of APEC infection emerged among in-line operations in the Midwest during the mid-1990s. The condition can cause up to 15% losses after peak production. Flocks may also show APEC peritonitis when molting and through onset of the second cycle of production. Erosive mortality under 2% over a few weeks is not generally recognized as APEC peritonitis unless routine post-mortem examinations are performed. In severe and recurrent episodes with cumulative flock mortality exceeding 10%, structured necropsy surveys will reveal the cause of losses
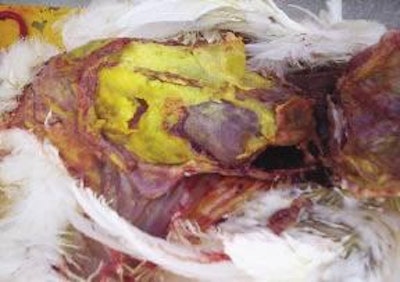
The pathogenesis of the condition has not been determined but it is assumed that inhalation of dust contaminated with APEC results in deposition of the organism in the abdominal air sacs. Local infection extends to the adjacent peritoneal surfaces and the serosal membranes surrounding the intestines, liver and reproductive tract, culminating in extensive peritonitis.
During the acute phase of infection, mortality increases from a normal level of less than 0.15% per week to over 1.5% per week. Most of the mortality comprises hens which are well fleshed, indicating the rapid onset of the infection and the development of acute septicemia. Characteristic lesions are obvious on examination. Flocks affected at or following peak production frequently show persistent elevated mortality.
Secondary APEC airsacculitis
Susceptible flocks which are exposed to respiratory pathogens including MG, ND, IB, LT and coryza, where prevalent, frequently develop secondary airsacculitis and septicemia as a result of APEC infection. The severity of the primary infection is influenced by the effectiveness of previous vaccinations, immune status, ventilation, environmental stress and nutrition. Pullet or mature hen flocks may show up to 10% losses. Generally, affected flocks develop secondary airsacculitis approximately one to two weeks after an obvious clinical infection characterized by respiratory rales (gasping and snicking) and if in lay, a depression in hen-week production. Post-mortem changes are obvious, involving caseous deposits in the abdominal and sometimes the thoracic air sacs. Post mortem examinations may also show concurrent peritonitis.
APEC salpingitis
Floor-housed hens and occasionally cage-housed flocks may show salpingitis (infection of the oviduct) late in the first cycle and during the second cycle. In caged flocks, affected hens will frequently die during molting or are culled at the onset of molt, consistent with good production practice.
Affected hens are emaciated and are out of production as denoted by shrunken combs and contracted pelvic conformation. Post-mortem examination reveals gross distention of the oviduct with caseous material and the condition is frequently associated with peritonitis and the presence of degenerating yolks in the body cavity.
Treatment of APEC infections
Flocks showing primary respiratory infection can be treated with one of the limited range of approved antibiotics for hens producing table eggs applying the FDA Prudent Use Principles. Drugs can be administered in either drinking water or feed in accordance with statutory label requirements.
Attempting to treat mature flocks with antibiotics is often unproductive and unjustified on a cost-to-effectiveness evaluation. Some success has been achieved in reducing mortality in pullet and young hen flocks by administration of mannanoligosaccharide feed supplements. These compounds function as prebiotics and stimulate tissue immunity in the intestinal tract.
Financial impact of APEC peritonitis
Financial losses associated with an episode of APEC peritonitis can be calculated using realistic assumptions related to standard production and mortality characteristic of infection. TABLE 1 documents the assumptions applied to calculating losses in a caged flock, including projections of egg production during the first and second cycles, standard mortality, nest-run average revenue of $1 per dozen and an assumed production cost of 70 cents per dozen.
In the specific example it is assumed that APEC mortality in the flock attains 5% by week 45 of production. It is calculated that a flock of 100,000 hens would lose approximately five eggs per hen on average during the first cycle and seven eggs during the second cycle following earlier mortality.
Adjusting costs of production to exclude the feed which would have been consumed by dead hens and altering the contribution margin accordingly, the loss of the equivalent of one dozen eggs per hen due to post-peak mortality in the flock of 100,000 hens would amount to $27,000 over two cycles. If losses due to APEC peritonitis occurred during molting, the loss during the 30-week second cycle in a flock of 97,000 hens would be $15,277. See TABLE 2 .
Airsacculitis and septicemia following secondary APEC infection could result in 2% mortality as shown in the example documented in TABLE 3 . Losses in an affected flock of 100,000 hens started would amount to the equivalent of 14 eggs per hen spread over the entire production period, attaining a value of $32,141.
Infection of pullets with APEC during the mid to second half of the growing cycle might result in a cumulative mortality of 2%. With a unit cost of $3.50 per bird the loss for a flock of 100,000 chicks started would be $7,000. Generally flocks affected with respiratory disease and secondary APEC airsacculitis show a proportion of pullets that are retarded in development and onset of production can be delayed by four weeks for up to 5% of the flock.
This may result in the loss of 12 eggs per affected hen or 0.6 eggs per hen averaged over the entire flock, amounting to $1,500. If pullets which die during rearing cannot be replaced with surplus hens, the 2% loss will result in a decrease in contribution margin from the 2,000 fewer hens placed. The flock operator would carry the same fixed costs but would not bear the depreciation cost from pullets purchased or feed consumed. The loss of these potentially producing hens would depress average flock yield by eight eggs per hen for a total of $18,366. See Table 3.
Prevention of APEC peritonitis
Programs which have been implemented in Midwest in-line operations are based on a presumed understanding of the causation and pathogenesis of the condition. Required measures relate to improving hygiene and biosecurity and protection of flocks from infection by vaccination.
Chlorination of drinking water to a level of 2 ppm at the point of entry to the house is a recommended practice irrespective of the E. coli status of the source.
Drinking lines should be flushed and biofilm removed with an acid detergent. Ventilation must be upgraded to acceptable production standards to suppress ammonia below 20 ppm for any period exceeding two hours.
Under conditions of extremely low humidity (30%) functional evaporative pads or ultra-high pressure fogging systems can be activated for short periods to raise humidity while maintaining a low rate of air flow.
Adequate air movement is critical to reduce accumulation of ammonia, carbon dioxide, and dust which stress the respiratory tract. Blowing dust from walkways and other house surfaces using compressed air or gasoline powered leaf-blowers should be stopped. Dispersing dust laden with APEC into the air of the house increases the level of exposure of flocks to respiratory infection.
Rodent suppression should be intensified to reduce contamination of the environment of hens with aerosolized APEC shed in droppings.
E. coli vaccination
Flocks of both caged and floor-housed hens in locations historically affected with APEC salpingitis, peritonitis, and airsacculitis have been previously vaccinated with a homologous-strain autogenous inactivated vaccine. These products are expensive to produce, require individual administration by injection and have variable effectiveness given the variety of APEC strains to which flocks may be exposed.
Advances in molecular biological technology have facilitated the production of gene-deleted bacterial pathogens including APEC strains. These vaccine strains lack pathogenicity and do not revert to virulence but can stimulate local tissue (cellular) immunity and humoral (circulating antibody) immunity. The first USDA licensed E. coli vaccine for chickens is based on an O78 strain of APEC which was modified to delete the aroA gene necessary for metabolism of cyclic amino acids.
Poulvac E. coli, manufactured by Fort Dodge Animal Health, has been used to suppress various forms of APEC infection in the U.S. egg industry. The vaccine should be administered within the first three weeks of age by the coarse spray route. The second dose should be administered at approximately 12 to 14 weeks of age as a booster. Extra-label administration by the coarse spray route has been used by some companies when initiating molting to protect flocks during the second cycle.
Experimental data submitted to the U.S. Department of Agriculture to support registration, and subsequent field evaluation have confirmed the efficacy of the vaccine. Given an approximate cost of $10 per 1,000 doses, a producer would invest $2,000 in vaccinating a flock of 100,000 pullets. If successive outbreaks of APEC peritonitis generate losses of $30,000 per flock, vaccination provides a potential benefit-to-cost ratio of 15:1. Suppressing airsacculitis mortality in pullets would provide a benefit to cost ratio of 8:1.
General practice is to administer E. coli vaccine to floor-housed pullets since their value at the time of placement, especially if reared according to organic rules, obviously justifies protection. Floor-housed flocks producing eggs for the organic and the non-confined markets generate a proportionately higher contribution margin than caged-housed hens and the attenuated gene-deleted live E. coli vaccine would generate a higher return especially with a history of peritonitis or airsacculitis in previous flocks.
The assumptions used in Table 1 for a caged flock and the projection of contribution margin as influenced by market factors as shown in Table 2 and Table 3 can be changed to suit specific market and production situations. The justification for protection against APEC peritonitis and airsacculitis increases proportionally to the value of flocks and contribution margin.






.jpg?auto=format%2Ccompress&fit=crop&h=167&q=70&w=250)











