
Egg producers have invested heavily in the past to reduce the incidences of Salmonella enteritidis (SE) in table eggs with refrigeration, improved cleaning and disinfection of facilities, enhanced biosecurity, better pest control, testing and record-keeping. An additional option that has widely been included in SE control programs has been the use of live Salmonella vaccines and inactivated bacterins.
"The low incidence of reported Salmonella enteritidis outbreaks from eggs in recent years is a testament of the success of the egg industry's efforts to control this organism," said Dr. Jim Sandstrom, managing director, Epitopix.
Dr. Kalen Cookson, director of clinical research at Zoetis, explained the rationale of using a combined live and inactivated vaccination program.
“As we can’t administer inactivated bacterins early in life, live vaccine has value to establish an early onset of local secretory antibodies (immunoglobulin A, or IgA) and cellular immunity in the intestine," he said. "We feel competitive exclusion as well as local immunity helps prevent the establishment of wild type Salmonella in the gut. There is also a potential of cross-protection against strains of other serogroups, which is also important. A second live (booster) vaccination increases both the level and duration of immunity."
"Inactivated bacterins induce long-persisting systemic (immunoglobulin G, or IgG) antibodies which circulate in the bloodstream. These systemic IgG antibodies provide a wall of defense by creating a barrier against invasive Salmonella from spreading to the reproductive tract," Cookson said.
Studies have shown the combination of live and inactivated Salmonella products provide the widest range of cross-protection over the duration of a layer’s production period. Scientists generally agree that conventional inactivated Salmonella products stimulate long-term specific immunity.
Greg Rennier, president of Rennier Associates, conducts an annual survey in which a representative sample of egg producers report their Salmonella vaccination programs, and those responses are projected to represent the total U.S. layer flock.

In 2018, 85% of the projected layer population received either two or three live vaccinations followed by an inactivated bacterin.
Live vaccines
Two live Salmonella typhimurium (ST) vaccine strains are available on the U.S. market, with three different products offered. Label claims all mention the reduction of Salmonella colonization in different organs with the approved vaccination program. Cross-protection has been shown to reduce incidences of S. enteritidis, typhimurium and Heidelberg. All are licensed for spray at day of hatch and drinking water application or coarse spray at later ages.
Poulvac ST offered by Zoetis is recommended for use at day of hatch and during grow-out. Elanco sells two live vaccines which contain the same strain with different formulations. Sandra Aehle, an associate adviser with Elanco, reports that AviPro Megan Vac 1 is recommended for day-of-hatch use and AviPro Megan Egg is recommended during grow-out.
Industry professionals agree that most producers apply the first live vaccine at day of hatch with a coarse spray application. Subsequent live vaccines are given in the drinking water or by coarse spray.
All live Salmonella vaccines licensed in the United States are derived from strains attenuated by genetic modification to ensure their safety and not cause Salmonella in vaccinated birds.
The National Organic Standard Board will be meeting in late October 2019 to decide if the board will continue to approve the vaccines developed with excluded methods (which includes genetic modification) for use in birds certified as organic.
Recently, a subcommittee of the board released a proposal to allow these excluded method vaccines to be used in organic production when an equivalent product made with conventional technologies is not available.
Inactivated bacterins
U.S. Department of Agriculture has licensed Salmonella enteritidis bacterins or combinations are offered by Ceva, Elanco, Merck and Zoetis. Generally, a combination product containing Newcastle disease, infectious bronchitis and Salmonella is given to pullets about three to four weeks before lay or transfer to the lay house.
Most producers administer the product intramuscularly in the breast muscle. Some producers have found subcutaneous injections of bacterins in the inguinal fold to be safer and less reactive for pullets.
Several manufacturers also offer production of autogenous bacterins to producers who desire specific immunity against a Salmonella strain that is not commercially available. As federal regulations require that the strain be isolated from the layer producer's farms, it is not common to have autogenous products produced unless there has been an outbreak of a unique Salmonella serovar.
Epitopix is producing autogenous Salmonella bacterins using a patented technology called SRP (siderophore receptor and porin), which isolates proteins common to all Salmonellas. This technology offers the potential to reduce the disease impact of serogroups other than those used to produce the bacterin.
Andy Long, layer business manager with Ceva, said the company has two unique adjuvant technologies in their product line besides traditional water-in-oil adjuvants. Autogenous products are now approved to use a lipid and polymer matrix adjuvant, ENABL, which is designed to invoke the desired immune response and reduce post-vaccination reactions.
Cevac Salmune TEK, a product used in the broiler breeder industry, uses a combination of water-in-oil-in-water and aluminum hydroxide adjuvants. This bacterin, containing three strains of serogroups B, C, and D, have been shown to provide protection against Salmonella typhimurium, enteritidis, Kentucky and Heidelberg strains. Egg producers that want to broaden their Salmonella coverage have this option to consider.
Post-bacterin syndrome
Industry professionals have observed severe reactions in pullets after administration of an inactivated bacterin. The syndrome is commonly titled post-bacterin syndrome or scientifically referred to as hemorrhagic hepatopathy. Dr. Eric Gingerich, technical services specialist at Diamond V, first reported on this condition in his annual review of layer health problems, referencing a case from 2004.
Mortality has been reported ranging from 1% to 7% in affected flocks, generally white egg layers. Some farms seem to have a higher incidence, and veterinarians are not able to link the problem with a specific manufacturer's bacterin. Some reports include clinical signs such as lame birds, diarrhea and convulsive-type movements.
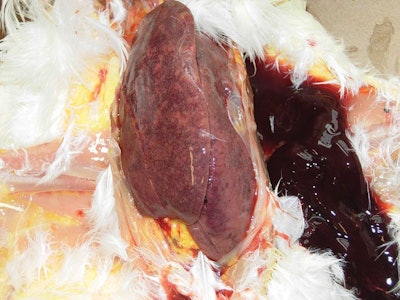
A common lesion found in birds with post-bacterin syndrome is an enlarged mottled liver that is friable (easily crumbled) and often results with hemorrhage present. (Dr. Eric Gingerich)
The most observed lesion is a liver that becomes enlarged, mottled in color and breaks down easily, resulting in hemorrhaging. Free blood can be found in the intestine. Even when the bacterin is given intramuscularly in a hen’s thigh, liver lesions are most common, which removes the theory of improper bacterin administration as the cause of the liver lesions.

Pale spleen and blood in the intestine are two other lesions observed with post-bacterin syndrome. (Dr. Eric Gingerich)
Clear answers are still eluding field veterinarians and researchers as to the source of this syndrome. Possible causes include high endotoxin levels in bacterins, overheating of the bacterin before administration or possibly an auto- or hyper-immune response.
While there are more than 2,500 serotypes of Salmonella reported around the world, fewer than 100 are responsible for most human infections. However, there is still much to be learned about those serotypes, their presence in poultry and their ability to contaminate eggs and egg products. Producers need to use all available tools to reduce Salmonella infections in layer flocks. Vaccination will continue to serve an important role in those efforts.















