There are many forms of metal complexes available in the marketplace for use in animal nutrition. These have been generically titled "organic trace minerals" by virtue of the fact that the trace elements in question are complexed or otherwise associated with organic molecules.
The chemistry of complexation, or chelation as it is commonly known, has created a great deal of confusion in the animal-feed industry. Terms such as metal amino acid complexes, metal amino acid chelates, metal polysaccharide complexes and metal proteinates abound, yet official definitions remain vague and unhelpful. As an example, the various definitions of organic trace metals used in agricultural practice as laid down by the Association of American Feed Control Officials (AAFCO, 1998) are illustrated in Table 1.
Given the vague definitions and confusion surrounding the precise physical and chemical nature of organic minerals, we first need to distinguish between the terms complex and chelate.
Complexes or chelates
Generically speaking, the term "complex" can be used to describe the species formed when a metal ion reacts with a molecule or ion (ligand) that contains an atom which has a lone pair of electrons. Such metal ions in a complex are bonded to the ligand through donor atoms such as oxygen, nitrogen or sulphur. Ligands with only one donor atom are termed "monodentate," whilst those that contain two or more donor atoms capable of bonding to a metal ion are termed bi, tri or tetradentate. These multi-donor species can also be referred to as polydentate.
When such ligands bond to a metal ion via two or more donor atoms, the compIex formed contains one or more heterocyclic rings which contain the metal atom. Such complexes are termed as "cheIates" (from the Greek; chele, a crab's claw).
Amino acids are examples of bidentate Iigands which bond to metal ions via an oxygen of the carboxylic acid group and the nitrogen of the amino group.
In contrast, ethylenediaminetetraacetic acid (EDTA) is an example of a hexadentate Iigand, which contains six donor atoms. It forms highly stable complexes with most metal ions and is, in fact, not particularly useful for the formation of mineral chelates as the bioavailability of such complexes is negligible.
Although cheIates having four, five, six or seven-membered rings may be formed, it has been shown that chelates having five-membered rings have the greatest stability.
It must also be remembered that even though all chelates are complexes, not all complexes are chelates. Indeed, whilst the overall theory behind chelation is simple, there are a number of criteria which must be absolutely met in order to ensure the generation of a stable mineral chelate.
A chelating ligand must contain two atoms capable of forming bonds with the metal ion.
The ligand must form a heterocyclic ring with the metal as the closing member of the ring.
It must be sterically possible to chelate the metal. The ratio of the ligand to the mineral must meet minimum requirements for stability.
True chelates have the "ring structure" formed by the coordinate covalent bond between the amino and carboxyl ends of the amino acid and the metal ion.
Typically speaking, chelates are prepared by reacting inorganic mineral salts with, for example, enzymatically prepared mixtures of amino acids and small peptides, under controlled conditions. Such amino acid and peptide ligands bind the metal at more than one point, ensuring that the metal atom becomes part of a biologically stable ring structure. Amino acids and protein digestion products such as small peptides are ideal ligands because they have at least two functional groups (amino and hydroxyl) allowing for the formation of a ring structure with the mineral. Only the so-called "transition elements" such as copper, iron, manganese and zinc have the necessary physico-chemical characteristics which allow them to form coordinate covalent bonds with amino acids and peptides, and thus, to create biologically stable complexes.
Amino acids and peptides as ligands
There are many different assertions made as to the relative merits and suitabilities of amino acids versus peptides in forming mineral chelates, with an even greater number of arguments existing in relation to the so-called bioavailability of such products. We have already considered the general criteria necessary for generation of a biologically stable mineral chelate; however, one must also consider a number of additional factors involved in the chelation process, the main aspects of which include:
- The relative equilibria — involving the metal ion and the ligand
- The kinetics of the substitution reactions of the hydrated metal ion and the complexes present.
- The redox behaviour of the metal ion and its complexes.
- Reactions involving the coordinated ligands.
Obviously, to oversimplify such a complex chemical phenomenon would be amiss.However, in order to dispel some of the myths surrounding the suitability of amino acids versus peptides in relation to mineral chelation, we will mainly consider factors affecting the equilibrium and stability of such complexes.
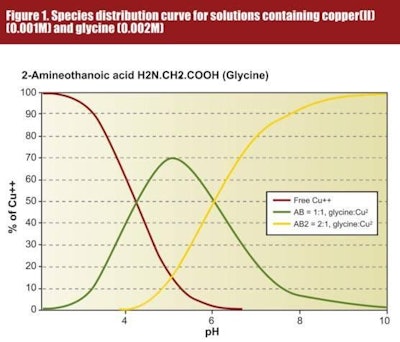
When a metal salt such as copper(II) sulphate is dissolved in water and a bidentate ligand such as an amino acid is added, a series of complexes will be formed, each of which have stability constants which are dependant on the solution pH. This is illustrated in Figure 1, where copper(II) sulphate has been reacted with glycine and from which a number of significant features can be highlighted:
- The distribution of metal species present at given concentrations of metal and amino acid depends on the pH of the solution.
- Chelates of the dipositive metal ions are not necessarily neutral.
- Different metal ions have different stability constants and thus, the percentage of a metal prsent as a particular species will depend not only on the pH of the solution but also on the stability constant of the complex.
The stability of metal complexes will ultimately depend both on the metal ion and also on the ligand. In terms of the effect of the metal ion on stability, increasing ion charge, decreasing size and increasing electron affinity will all result in a stabilising effect. The ligand also has several characteristics which are known to influence the stability of complexes: (1) basicity of the ligand, (2) the number of metal-chelate rings per ligand, (3) the size of the chelate ring, (4) steric effects, (5) resonance effects and (6) the ligand atom. Since coordination compounds are formed as a result of acid-base reactions, where the metal ion is the acid and the ligand is the base, it follows that generally, the more basic ligand will tend to form the more stable complex. The size of the chelate ring is likewise an important factor.
Considering Figure 1 even further, one can begin to appreciate that there are distinct differences between the relative stabilities of metal amino acid chelates and metal proteinates. Given that a metal proteinate is the product resulting from the chelation of a soluble salt with amino acids and/or partially hydrolysed protein, it should be anticipated that for a given metal ion, the complexity of the species distribution curve for the proteinate will be far greater than for the corresponding metal amino acid chelate. If we think of the species distribution curve as being an indicator of relative stability at given pH, and bearing in mind the infinite combinations accruing from a mixture of single amino acids in conjunction with di, tri and even tetrapeptides, then the overall proteinate stability over a wide pH range should in theory be far greater than for a specific metal amino acid chelate.
Biological stability
Obviously, the additional influencing factors discussed earlier will ultimately contribute to a chelate's overall stability under practical conditions. In relative terms, however, one would anticipate that metal proteinates will have the necessary physico-chemical properties to ensure wide-ranging constancy under conditions of changing pH.
Despite the confusion and often contradictory information that exists, mineral chelation is a relatively straightforward process governed by some fundamental chemistry basics. In general, we can distinguish two true forms of mineral chelate, each of which have defined chemical and biophysical properties. By carefully considering factors important in mineral chelation, one can begin to distinguish between the products on the basis of biological stability and thus, biological bioavailability.