Experienced gained in the EU and the U.S. has demonstrated the value of vaccines as a critical component of an integrated program to prevent vertical transmission of Salmonella enteritidis (SE) from infected flocks to consumers. The introduction of the FDA Final Rule followed by the August 2010 SE recall emphasizes the need for diligent and effective suppression of infection. The principles of control include:
- Purchase of chicks from a hatchery compliant with the “SE Clean” regulations;
- Effective biosecurity measures;
- Intensive suppression of rodents; and
- Successful immunization using approved vaccines.
Based on surveys it is believed that from 5% to 10% of U.S. flocks may be infected with SE. The Centers for Disease Control estimate that there were approximately 110,000 egg-attributable cases of SE among consumers in the U.S. in 2007. From 1985 to 2007 there were 376 outbreaks of SE in which eggs or egg products were implicated, involving 18,000 patients. Although the numbers of outbreaks caused by egg-borne SE have decreased from 1990 (85) through 2007 (29) concern over SE by successive federal administrations and public health officials has resulted in the Final Rule on salmonella introduced in July 2010. Concern for SE among consumers is evidenced by the sharp drop in consumption following the mid-August recall which seriously impacted margins and profitability. Losses to the industry during September alone were estimated at $125 million based on the difference between the projected UB price and actual realization.
Following the emergence of SE as an egg-borne infection of consumers, biologics manufacturers applied two lines of development in producing and marketing salmonella vaccines. In the EU, producers adopted inactivated oil emulsion vaccines in 1994, which qualified for rapid licensing but required injection into individual pullets. The second approach was to develop attenuated avirulent mutant salmonella vaccines which would offer protection when mass-administered to flocks either in water or by the aerosol (spray) routes.
The innovative nature of these live vaccine strains required extensive laboratory and field testing to convince regulatory authorities of their effectiveness and safety. Additional restraints arose from claims relating to intellectual property and the patent status of both products and the technology used to develop gene-deleted and mutant strains. Although these factors delayed release, both commercially available live mutant salmonella strains and inactivated emulsions are available and have a complimentary role in integrated SE prevention programs.
Attenuated mutant vaccines
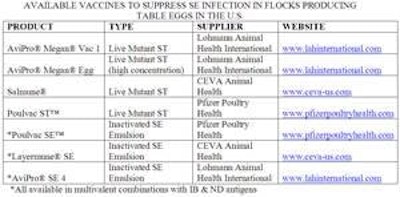
The three commercial vaccines (see chart) licensed by USDA are based on Salmonella typhimurium which has been modified by either deletion of genes or exposure to media containing chemical compounds which induce mutations.
- Lohmann Animal Health market AviPro® Megan® Vac 1. This is an attenuated ST containing a natural occurring plasmid which is stable and due to size (91 kb) is not regarded as being potentially transmissible other salmonella or related genera of enterobacters. Mutations in the cya and crp genes affect the internal metabolism of the modified salmonella, suppressing the ability to reproduce. The mutant is deprived of pathogenicity compared to the wild-type progenitor organism. The vaccine strain retains the ability to bind to receptor sites on enterocytes lining the intestinal tract. The vaccine strain serves as a highly specific competitive exclusion organism against naturally occurring salmonella including SE and paratyphoid serotypes including Heidelberg, Typhimurium, Kentucky and Hadar among others. The vaccine strain can also invade gut-associated lymphoid tissue and stimulate significant local immunity. Extensive studies have failed to demonstrate any reversion to the wild-type state.
- Lohmann Animal Health distributes AviPro® Megan® Egg which incorporates a higher concentration of the vaccine strain and is intended for the second or pre-production dose for pullets to enhance protection against SE.
- Pfizer Animal Health PoulVac ST vaccine was developed and marketed by Fort Dodge Laboratories prior to its acquisition. The strain is a mutant with deletion of the Aro-A gene. This prevents the organism from producing aromatic amino acids (tryptophan tyrosine and phenylalanine) required for reproduction and other biochemical functions.
- CEVA Salmune™ is a Salmonella typhimurium mutant derived by chemical treatment of a wild-type strain.
Mutant ST vaccine strains do not generally persist in the intestinal tract beyond 21 days following the initial day old to 48-hour administration. This necessitates administration of a second dose at 14 to 21 days to ensure protection of receptor sites until a robust competitive intestinal flora develops.
None of the mutant ST vaccines elicits a humoral antibody response and it is not possible to detect circulating antibody following vaccination. The ST vaccines will not produce a false positive reaction to the whole-blood plate agglutination test for Pullorum disease.
The mutant ST vaccines can be administered at the hatchery using an aerosol vaccination cabinet. Alternatively vaccines can be delivered to newly-placed flocks by coarse spray or in drinking water provided appropriate precaution are taken to ensure even distribution and to prevent inactivation by chlorine. The second dose is usually administered in the drinking water or by coarse spray applying precautions as directed by the manufacturer. Some producers apply a third vaccine to their pullets at 10 to 14 weeks, frequently selecting the more concentrated product. The three mutant ST vaccines as well as Megan® Egg may be administered off-label to producing flocks in an attempt to limit intra-flock spread of SE in the event of introduction of infection by rodents or defects in biosecurity.
Pre-registration trials to demonstrate safety and efficacy of ST mutant and gene-deleted vaccines have shown significant reduction in colonization rates within the intestinal tracts of chicks receiving vaccine when subsequently challenged with field strains of SE and other Salmonella spp. Approximately 10% to 30% of vaccinated chicks may excrete SE after high levels of challenge (108 organisms) under controlled laboratory conditions. In cleaned houses, level of exposure is relatively low and it is presumed that if adequately vaccinated flocks are exposed, the extent of intestinal colonization will be proportionately lower compared to deliberate exposure under laboratory conditions.
In the context of the U.S., certain practices have been implicated in reducing the efficacy of ST vaccines. The most significant factors relate to incorporation of antibiotics in the diluent used to administer Marek’s disease. Studies conducted by Megan Health Inc., the developer of Megan® Vac were conducted with Naxcel™ (sodium ceftiofur) penicillin G and gentamicin. Day-old administration of Naxcel™ did not materially interfere with colonization of the liver, bursa of Fabricius or cecum with the Megan® Vac 1 vaccine. Slight reduction in recovery from all three sites was observed on the sixth day post vaccination in chicks receiving 0.2mg Naxcell™ at day old. There was no appreciable effect observed following administration of penicillin (5,000 units by the subcutaneous route). Generally, it can be concluded that the dose levels of antibiotics currently used will not interfere with the effectiveness of gene-deleted or mutant ST vaccines administered at the hatchery.
The second consideration concerns incorporation of organic acids in feeds to suppress proliferation of salmonella which may contaminate ingredients. Adding propionic acid to feed to reduce the pH value to approximately 3.0 units had no effect on the colonization of the bursa, cecum or liver following vaccination with Megan® Vac. when assayed at one day and seven days after administration.
Studies conducted by Alltech have been conducted to show that administration of their mannanoligiosaccharide prebiotic Bio-Mos®, known to agglutinate organisms with Type-1 fimbriae did not have any adverse effect on mutant ST vaccines.
It is considered likely that the benefits of a vaccination program comprising a day-old to 24 hour administration followed by a second dose during the period 14 to 21 days can be enhanced by simultaneous administration of a probiotic in either feed or water.
Studies published in peer-reviewed journals confirm that mutant ST vaccines do not prevent systemic infection with SE, which may result in colonization of the liver, ovary, oviduct with inconsistent contamination of egg pools. The live mutant and gene-deleted ST vaccines are regarded as a method of protecting the intestinal tract from environmental contamination during the early brooding period. The mutant and gene-deleted ST vaccines stimulate effective tissue immunity but of relatively short duration and with minimal protection from vertical transmission to eggs subsequent to transfer of pullets to potentially contaminated houses or complexes.
Inactivated emulsion vaccines
Inactivated emulsion vaccines have been used to control paratyphoid salmonella for many decades. The efficacy of autogenous vaccines has been demonstrated in specific breeding operations where paratyphoid strains have become endemic. In countries where “fowl typhoid” (Salmonella gallinarum) is prevalent, it is common practice to apply programs incorporating both live and inactivated emulsion vaccines.
With the emergence of SE in the EU during the 1980s, inactivated SE vaccines were prepared and deployed rapidly to reduce rates of infection of flocks and to reduce vertical transmission. A number of studies in peer-reviewed journals confirm the efficiency of SE emulsion vaccines. Studies conducted in Holland, Germany and Japan demonstrated reduction and in some cases elimination of egg transmission in flocks placed on farms which previously housed infected flocks responsible for vertical transmission.
Experiments conducted in the U.S. by the USDA-ARS Southeastern Poultry Research Laboratory confirmed a high level of protection but not absolute elimination of vertical transmission following administration of oil emulsion vaccines. These trials involved high levels of challenge administered under laboratory conditions. Oil emulsion vaccines stimulate antibody which can be measured by commercially available ELISA assay.
Vaccination programs
Although no single program can address the needs of all producers, one approach (outlined in this chart) is generally used in the U.S. egg production industry.
Variations of this program may be followed by individual producers. Faced with SE environmental positive housing these may include the use of two emulsion vaccines or extra-label administration of high concentration mutant ST prior to molt. Administration of live mutant ST vaccine during the laying period will not reduce the probability of detection applying environmental assays. Administration of inactivated emulsion vaccine prior to transfer will reduce the probability of vertical transmission in the event that flocks are exposed to SE. Flocks with circulating antibody when exposed to SE from infected populations of mice would most probably result in a positive environmental assay but a negative egg pool. It is noted that the level of transfer of SE into eggs is low (10 to 100 cfu). This is the reason for rapid cooling to 45F which inhibits proliferation to attain an infective dose for consumers even if eggs are not adequately cooked.
Contaminated eggs subjected to thermal abuse may have upwards of millions of SE organisms within 10 days representing a public health hazard. Vertical transmission from infected hens is sporadic but stress associated with molting or immunosuppression can markedly increase the prevalence rate of infection. A combination of subjecting eggs to room temperature, prolonged storage at above 45F and starvation molting will overwhelm the benefits of vaccination and lead to SE outbreaks in consumers. The conventional microbiological procedure to detect SE in egg pools is generally regarded as being of low sensitivity. In contrast application of PCR technology results in higher sensitivity and specificity and a result can be obtained within 24 hours from receipt by a laboratory.


















