The modern pen is likely to contain fixtures or fittings of stainless steel, plastic and even glass. Fortunately, all of these can bear any modern formulated detergent used for cleaning purposes on a farm, provided that the correct dilutions are used. More issues arise in the case of concrete (cement). Along with copper and brass, it cannot stand strong acids. Also, galvanised steel is quite sensitive to acid products.
This in itself will influence the choice of a detergent to use as a cleaning agent. But our experience has been that the biggest challenge in cleaning pig-pen materials comes from the use of aluminium. It can be found in various places in farm buildings (usually as aluminium frames and profiles) and on the trucks or trailers employed in hauling animals. Treated badly, however, it does not stay intact.
Aluminium surfaces or equipment cannot withstand most alkaline detergents, being those based on sodium hydroxide ('caustic soda') or potassium hydroxide. Even more, they are especially not resistant to chlorinated detergents — the ones based on sodium hypochlorite or 'bleach'. Applying such cleaners to aluminium causes the so-called white spots, a form of corrosion. In some other cases the detergent turns the aluminium black.
However, aluminium can stand acids. On cars, it is standard practice to use acids to clean aluminium wheel rims. The problem in practice at pig units is that acid detergents do not make a good job of removing organic dirt. To remove pig dung, you need an alkaline detergent.
There is the middle option of using a detergent that has a neutral pH. The cleaner will not affect the aluminium, yet it will be better than an acid at removing manure. But it is not ideal for the removal of organic matter. The recommendation we make is to use a non-chlorinated alkaline detergent so the dirt on pen surfaces is removed well, while making sure to choose a product of that type which has special corrosion inhibitors built-in to protect aluminium.
Although our advice is always to pick a detergent with the right pH to remove pig manure, therefore, a further consideration will be whether you have any aluminium equipment on your unit. Ask your supplier if the alkaline cleaners being offered can be used on aluminium. You may extend the lifetime of your precious equipment considerably!
It is just one example of the basic fact that chemistry is important in cleaning. Your choices of cleaners should reflect not only their ability at removing different types of dirt, but also an understanding of the type of surface material on which certain chemicals should be avoided.
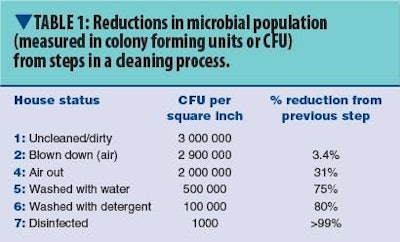
A thorough clean-out of a pig pen must begin by removing the dirt that likely harbours many microbes. Only then can a disinfectant do its job. Table 1 gives results from investigations done in the USA at the poultry research farms unit of North Carolina State University. They established how an initial micro-organism population had been reduced by each step of cleaning and disinfection.
Four factors determine the functioning of a detergent. In view of the above discussion about pen materials, the first of these needs particular emphasis because it relates to the product's chemical energy, pH and concentration. The science says that alkaline detergents — those with a pH above 8.0 — remove proteins and fat or organic dirt. Acid detergents — meaning those whose pH is under 6.0 — remove mineral deposits such as limescale and inorganic dirt.
Thermal energy is the second factor and it refers to the fact that fat starts to dissolve at a temperature of 35°C. Physical energy in detergent application comes from the use of a high-pressure washer. Then there is the vital consideration of contact time.
Foam detergents have become popular over more classic types because they adhere longer to the surface and therefore give a longer contact. An argument against them, on the other hand, is that they may eventually dry and leave a residual film which is difficult to remove. A new generation of cleaners answers that because the cleaners are in the form of a gel which does not dry at all, so its chemical action persists. PIGI

















