Salmonella and campylobacter continue to be leading causes of food-borne illness around the world, and they are often associated with poultry products.
While levels of salmonella can vary greatly depending on time of year, region, flock and environment, researchers have shown that when 3-4% of birds tested positive for salmonella enter a plant, 20-35% were positive after processing. Thus, cross-contamination is one of the hurdles to overcome to reduce salmonella. Cross-contamination is not easily controlled due to the level of automation and multitude of steps during processing. More importantly, if the pathogens are controlled on the farm, they do not enter the plant and cross-contamination is not an issue. However, most food safety controls are implemented at the plant.
To determine which steps pose the greatest risks, microbial testing and data analysis (biomapping) can be used to determine which farms are likely salmonella-positive and what processing steps pose the greatest cross-contamination problems. In addition, it is essential that a team with representatives from each phase of the production and processing system document food safety policies and practices and share these policies so they are communicated, understood and implemented throughout the system.
Multiple strategies
Multiple intervention strategies are needed to address salmonella throughout processing. Current strategies include physical and chemical interventions or a combination of the two. Good environmental stewardship would suggest that chemical use be minimised by implementing further physical intervention strategies. Their application needs to begin early in the process. Some of the first areas to consider are flock scheduling and cooling sheds.
Flock scheduling
If a facility employs biomapping and tracks salmonella, farms that are likely to be positive can be identified. A flock from a farm thought to be salmonella-positive should be processed towards the end of a shift to prevent cross-contamination that could occur if salmonella-positive birds are processed earlier. This approach takes co-ordination and communication in addition to assuming the company knows which farms (if any) are likely to be positive.
Cooling sheds
Since higher moisture promotes bacterial recovery, cooling sheds should be one of the first areas at the plant to be examined. During the summer, misting is often used as way to help keep birds cool. Providing too much moisture can increase the spread of bacteria and may have a negative effect on the birds' ability to dissipate heat.
Water quality
Water quality can affect antimicrobials as well as functional ingredients used throughout processing. Specifically, water hardness, pH and mineral content can alter the activity of antimicrobials. Water softeners can alleviate problems caused by water hardness. Iron content and other ionic minerals can also interfere with the action of antimicrobials. Water pH needs to be monitored so appropriate adjustments can be made depending on the application. For instance, water with a high pH will render chlorine ineffective. Plants need to understand the effect water quality has on antimicrobials, cleaning chemicals and functional ingredients, and to closely monitor the water quality and sources of water used.
Scalding, picking and evisceration
Brush scrubbers are a physical antimicrobial step that remove some of the foreign material and excreta from birds prior to the scalder. Chemical rinses, generally hypochlorous acid, can be added to increase the effectiveness of this step.
The scalder is generally thought to reduce bacterial loads because of the high temperatures. However, factors such as organic load, scalder configuration, water flow rate and counter-current flow, water pH and temperature can limit its effectiveness. High organic loads will act as a buffer in the chiller water, and salmonella grows at pH6.5-7.5. Using scrubber brushes and rinses prior to birds entering the scalder will reduce the organic load.
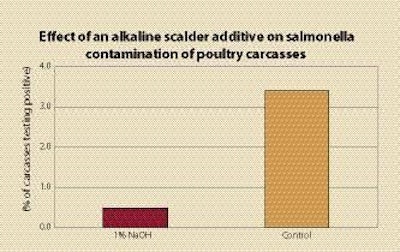
Scalder additives can be used to adjust scalder to pH 9 or above, or below pH 4.5 as a means of preventing salmonella growth. Organic acids such as acetic acid have been used to lower the scalder pH; sodium hydroxide (NaOH) has been used to raise pH. While acetic acid can be effective, it can cause a vinegar smell. It is effective as low as 0.1%, while NaOH is effective at 1%. Water has fewer solids with alkaline additives as they fall to the bottom, and birds appear cleaner. Salmonella and campylobacter were destroyed when carcasses inoculated with these bacteria were processed through the scalder containing the alkaline additive.
Scalder configuration
Many facilities have multi-stage scalding systems with counter-current water flow., i.e. birds move towards cleaner water upon exiting the scalder. Maintaining a high water flow rate is important to dilute organic material and bacteria.
Another factor influencing bacteria reduction in the scalder is the water temperature. While most facilities use a hard-scald process (55.6-57.8°C), some use a lower temperature or soft-scald process (48.9-51.7°C). Residual Salmonella heidelberg was found in a facility using a soft-scald process. S. heidelberg is more heat-stable than some other salmonella serotypes. Since the facility had implemented a biomapping programme where various sites in the plant were tested, it was determined that the S. heidelberg was neither destroyed by the scald temperature nor removed by cleaning and sanitising. Changes were made in the sanitary standard operating procedures to address this issue.
Further analysis helped to identify the farm where S. heidelberg had originated. A physical process implemented at this step was increasing the scalder temperature to 73.9°C during breaks or shift changes, and then bringing it back down prior to start-up. The higher temperature helped destroy bacteria that may not have been destroyed by the original scalding temperatures. While this physical step is very effective, it warrants close attention to make sure scald temperatures are correct before resuming processing.
Picking
During processing, the microbial load on picking fingers increases over time, and wear and tear lead to cracks and crevices. Bacteria here are easily removed during cleaning and sanitation so picking fingers should be checked and replaced regularly. Picking fingers should be effectively cleaned and sanitised daily.
During picking, chlorine sprays may be used to control bacterial counts but the chemicals can damage the picking fingers. Organic acids, such as acetic acid, are effective but the smell could be a factor. Washes pre- and post-picking have shown inconsistent results on the final bacterial load on poultry carcasses, but they may provide an overall dilution effect. Generally, when carcasses are rinsed post-pick, the most typical chemical used is chlorine at levels of 20-30 ppm. Other antimicrobials can be used as well if the plant configuration allows them to be pumped to all required areas.
Evisceration
During evisceration, the best practice in terms of food safety includes optimising feed withdrawal so the intestines are cleared but are not fragile and do not break during evisceration. Short withdrawal times have been shown to increase faecal contamination on processed carcasses but longer times (more than 12 hours) can cause the intestines to rupture due to fragility. Studies have shown that crop contamination with salmonella and campylobacter increases during pre-slaughter feed withdrawal, possibly due to birds consuming pathogen-contaminated litter.
To optimise feed withdrawal from a food safety stand-point, it is important to minimise litter consumption while meeting the 'zero tolerance' for faecal contamination on carcasses at the plant. Generally, 8 hours is recommended as an optimal feed withdrawal time to help meet the food safety criteria. Feed withdrawal may need to be further optimised based on the size of the bird. Bigger birds need more time for gut clearance than smaller birds.
Equipment needs to be properly adjusted and effectively cleaned and sanitised. Disinfectant rinses need to be checked to make sure the chemical levels, spray pressure and distribution are correct.
Bird washers and rinse cabinets
Rinse cabinets may be found at multiple locations throughout the plant. Rinse cabinets used for external bird rinses are often located at pre-scalding, picking and post-picking. Inside/outside bird washers (IOBW) are used after evisceration or prior to chilling.
Antimicrobials used may include acidified sodium chlorite, trisodium phosphate, peroxyacetic acid, chlorine dioxide, hypochlorous acid (20-30ppm), organic acids and cetylpyridinium chloride. Acidified sodium chlorite (Sanova, 500-1200ppm) is probably the most commonly used in the USA. Trisodium phosphate prior to the chiller may raise chiller pH to above 9, and if chlorine is being used, it will not form hypochlorous acid, which is the antimicrobial. Prior to the chiller, it is better to use antimicrobial rinses that have an acidifying effect, or at a minimum, no affect on the chiller pH.
Antimicrobials applied in spray rinse cabinets provide inconsistent results because of variations in contact time, concentration of the antimicrobial used, bird coverage (distribution) and spray pressure. Equipment operations that need to be checked include spray nozzle function, water pressure (nozzle) and spray coverage, which should be optimised with the line speed to ensure adequate contact time.
It is important to validate the antimicrobial being used to determine the effective concentration and to find out if it works for the application. IOBW may be used in automated on-line re-processing, which reduces the need for off-line re-processing and increases overall processing efficiency.
To view the table at full size, please click here











