Biofilm, a persistent threat to poultry drinking water, is an accumulation of organic and inorganic microorganisms bound by extracellular polymer substances that, without proper management, may pose a threat to flock performance in a variety of ways.
Growing on the water's surface, biofilm may form a 'slime' that adheres to pipes and storage tanks causing clogged drinkers, damaged equipment and reduced water flow. However, it can also directly threaten flock health, allowing microorganisms such as harmful bacteria, yeasts, and molds to contaminate drinking water.
There are four commonly used methods to manage biofilm threats in poultry drinking. The first addresses pathogens that enter the bird through contaminated water, while others focus on preventing pathogen intake. Combining all four methods presents a particularly effective approach.
1. Acidification supports digestion
Providing a level of protection against pathogens that enter the bird via the biofilm, acidification helps control water and stomach microbes while reducing pathogenic bacteria in birds' intestines.
Water acidifiers reduce the pH of water to less than 4 – a level at which many pathogenic bacteria struggle to survive. Low pKa (strength of an acid) and undissociated organic acids reaching the stomach may also help control gram-negative bacteria ingested through contaminated feed or feces. These organic acids may deliver antimicrobial efficacy in the acidic stomach region as they pass through the walls of bacteria and fungi, altering their metabolism.
As a low pH is required for the digestion of most plant- and animal-derived proteins, organic acids can help assure less undigested protein reaches the hindgut, potentially reducing the threat of dysbacteriosis.
The goal of using water acidifiers is to achieve a pH of approximately 3.8 to 4.0. Water can be classified according to its alkalinity, or buffer capacity - a measurement of water's capability to neutralize acids. Knowing the water's buffer capacity helps determine the best blend (level of buffering) and the recommended application rate.
In water with high buffer capacity (high alkalinity), a higher inclusion rate of acidifiers is required to reach this target pH. Less-buffered acidifiers can help reach the target pH at lower doses. A blend of organic acids may be worth considering when the objective is to combat biofilm and improve animal digestion, gastrointestinal microbial balance or animal performance.
Dosage is especially important. Higher amounts of a water acidifier will make more of the acid molecules available to the animal to support desired effects. A poultry nutritionist can advise on optimal dosing.
2. Flushing to dislodge biofilm and build-up
Clean drinking water begins with flushing. The system should be flushed with clean water between bird cycles and after any treatment, such as vaccines, antibiotics or vitamins delivered through water. As disease risk is highest during a bird's first week, it is advisable to flush systems at least twice during the first week of production.
Flushing with clean water loosens substances that can contribute to biofilm and washes away build-ups that can clog equipment. High pressure of 2 to 3 bars/units should be applied during the flushing process.
3. Hydrogen peroxide to disinfect between cycles
A simple and effective disinfectant between cycles, hydrogen peroxide works very well in killing many bacteria and removing biofilm build-up. It also helps prevent biofilm from accumulating.
Applying the proper concentration of hydrogen peroxide and allowing adequate treatment time is important to achieve desired disinfection results.
Hydrogen peroxide comes at different concentrations (35 to 50% hydrogen peroxide to water). Generally, applying 1 to 3% (1-3 L/100 L) water to a hydrogen peroxide solution is optimal but check with the supplier to confirm recommended levels. The hydrogen peroxide treatment can cause pressure inside the drinking lines to increase so a valve should be opened to relieve pressure. Allow the solution to stay in the system for 24 to 48 hours.
Following disinfection, it is very important to flush with clean water.
4. Chlorination as maintenance during cycles
Chlorine can be used as a maintenance disinfectant during the production cycle. Again, flushing is the first step in the disinfecting process, followed by chlorination.
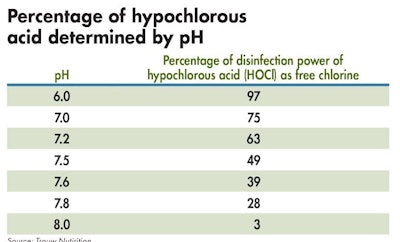
Chlorination becomes even more effective when combined with a proper approach to acidification. (Courtesy of Trouw Nutrion)
Generally, a concentration of sodium hypochlorite at 15% (100 to 150 mL/1000 L water) or chlorine dioxide applied at 0.2 to 0.4 mg/L water is recommended, but always check with the supplier to confirm levels.
The target level of residual free chlorine is important and should be between 3-5 ppm or oxidative reduction potential (ORP) in the range of 650 to 700 millivolts.
Measuring the ORP in the water helps assess the balance between the water's pH and free chlorine to assure water is effectively sanitized. ORP reflects the activity of the water sanitizer as opposed to its concentration level (ppm).
Chlorination becomes even more effective when combined with a proper approach to acidification. Chlorine reaction (HOCl ↔ OCl- + H+) is pH-dependent (Figure 1, Table 2). At a pH 5 to 6, the chlorine species is nearly 100% hypochlorous acid (HOCl) and highly effective at killing bacteria. As the pH drops below 4, it starts to convert to Cl2 (chlorine gas). Above pH 6, it starts to convert to the hypochlorite ion (OCl-) and acts mainly as an oxidizer. Water with a pH ≥ 7 may need to be lowered for chlorine to achieve optimal bacteria-killing efficacy.
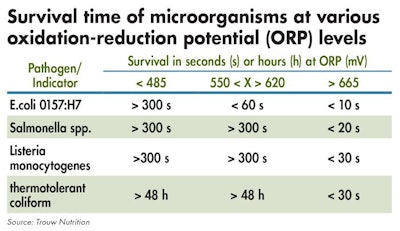
The oxidative reduction potential of water will influence micro-organism survival times. (Courtesy of Trouw Nutrition)
To learn more, read:
Efficient water management practices for broiler flocks
www.WATTAgNet.com/articles/35236
















