The USDA Food Safety and Inspection Service's (FSIS) proposed changes to the Salmonella Verification Sampling Program and related activities are tough on the poultry industry, tightening limits on salmonella levels and threatening to slow or stop processing lines if the new standards aren't met. The changes are broad, including the addition of in-plant testing to hunt for human illness-related serotypes, the finding of which could trigger incteased testing and enforecement action. Clearly, FSIS is getting tough on industry in its new policies, but are they the right proposals to help reduce samonella in poultry?
Proposal 1: Publication of Verification Results
FSIS plans to publish the completed verification sample set results for plants that show inconsistency in their ability to meet Salmonella Performance Standards, beginning with those from young chicken slaughter establishments. Posting of these results began on March 28, 2008. This measure will separate companies and, perhaps, plants within companies that are microbiologically superior to others. It may have a dramatic negative impact on sales for companies that consistently fall into the Category 2 and 3 ranges. Currently, no one poultry company has a widespread reputation for producing "significantly safer" products or chickens with lower salmonella prevalence. This rule may change that.
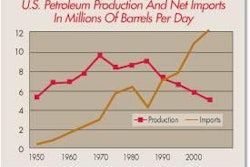
The Agency states that it is implementing the new rule "to address the adverse trends in salmonella percent positive samples seen in young chickens during 2000-2005." The years 2006 to 2008 are obviously absent from this statement. Why? A chart depicts data from these years, and it is clear that salmonella prevalence has dramatically decreased during the 2006-2008 time period.
Fallacy underpins rationale
Moreover, the decrease has been steady since 2005, and there is no question that salmonella prevalence across the USA is now hovering at the lowest point since the inception and implementation of the HACCP/Salmonella Performance Standard in 1996. This begs the question, Why is FSIS not giving the industry incentives to keep the numbers low?
A large proportion of U.S. chicken that is exported goes to countries such as Russia and China, which have a zero tolerance policy for salmonella on imported chicken. The method they use to detect salmonella is inappropriate and would certainly result in a negative result, the method FSIS uses is very sensitive and will often find a single salmonella cell. This could result in decreased poultry exports..
A fallacy underpins the Agency's rationale. Salmonella prevalence is not simply based on the processing plant's ability to control its process. Plants often run day in and day out in the exact same way, with no variances in chemical additions, water flows, temperatures or other microbial interventions that control salmonella. However, salmonella prevalence will go up and down based on season of the year, treatment of chickens using medications, diseases that occur in the flock, the amount of humidity in the air, and other factors too numerous to enumerate in this article. This reality is not reflected anywhere in the FSIS reports. It will not be mentioned in the table where the processors identity will be revealed to the public either. The customer comes away with an absolutely incorrect assumption that the plant is out of process control. With this in mind, why penalize the poultry plant for the weather, disease outbreaks, and other variables under the guise of "the plant lost control of the process"?
Proposal 2: Classifying Plants by Performance
FSIS proposes to classify establishments in three process control categories according to their performance in completed sample sets relative to the regulatory performance standard and potentially lowering these standards.
New scheduling algorithm
FSIS implemented this procedure in July of 2006 when a new scheduling algorithm went into effect. The Notice explained that a Category 1 "establishment will now be tested no more than once a year, but at least once every two years, unless it gets a result that puts it in Category 2 or 3" (71 FR at 9776). This algorithm has the effect of placing greater emphasis on scheduling young chicken establishments, which are currently the Agency's top priority. However, if the numbers for each category are reduced, the probability of a processing plant falling into a higher category is greatly increased, and the frequency of testing will be increased as well.
Also under this regulation the sample collection or the frequency of entering a ‘Set A' testing series is based on these categories. Increasing frequency of testing may not have the desired effect. In many cases, improvements to the plant cannot be made in the amount of time that FSIS gives the plant prior to retesting. Setting an arbitrary rescheduling of testing because of a mathematical formula does not take into account real-world problems faced by the industry.
The proposed changes to the regulation are shown in the Figure, "Current Vs. Proposed Categories." Before the proposed changes, a plant with 23.5 percent of carcasses positive for salmonella or greater would be penalized; whereas, if the standard is changed, plants with greater than 7.5 percent of carcasses positive for salmonella would undergo some punitive measure. It is estimated that many plants would be affected.
Proposal 3: HIMP, OLR Contingent on Category
The FSIS Salmonella Initiative Program (SIP) originally was known as the Salmonella Incentive Program because the USDA was to give the industry an incentive to keep salmonella numbers low. Plants were to be given the ability to increase line speeds if they kept their salmonella levels low enough. Now, only five plants will be able to participate, and they would be mistaken to fall into this trap. If the plant ever goes into Category 2 or 3, it will immediately have to reduce line speed. What would a plant do if it has enough chickens in the field to supply a 180 bird-per-minute (bpm) line speed and suddenly had to run at 70 or 90 bpm?.
Originally, it was to be voluntary. However, as written, plants that operate under the HACCP-based Inspection Models Project (HIMP) or Online Reprocessing (OLR) waivers, would lose their ability to process using the HIMP inspection program or using OLR systems if they go into Category 2 or 3. How would companies completely change out equipment and employees needed to go immediately from HIMP inspection to traditional inspection?
With regard to the OLR system, USPOULTRY wrote to FSIS for clarification as follows: "With regard to On-Line Reprocessing (OLR), will establishments be immediately required to stop using one of their major microbial interventions, and at the same time be expected to improve salmonella performance before being allowed to use this intervention again?" This is an excellent question and has no logical answer..
Plant microbiological testing
Processing facilities will be required to begin testing carcasses at rehang and post-chill for salmonella, campylobacter and E. coli. One immediate criticism of this approach is that no one has ever been able to demonstrate an adequate means of controlling campylobacter on chicken, especially at the rehang area. The cost for conducting campylobacter analyses is expensive (>$90 per sample if sent to outside lab) or very difficult and cumbersome if done in house. (see "Trends In Microbiological Testing Technologies" at www.WATTpoultry.com/0801USAtesting.aspx.
Serotypes: USDA will be evaluating the serotypes of salmonella on all positive carcasses. Two or more serotypes that are the same as those recovered from a foodborne illness (even if the illness was from peanut butter) will result in an immediate Cat 2 placement. Even if salmonella prevalence is Cat 1 (< 7.5 percent), if two serotypes from a foodborne illness causing bacterium are found, FSIS begins Set A. The more they test, the probability of failure increases dramatically. Again, no research exists that demonstrates that one serotype of salmonella may be controlled over another. In fact, salmonella serotypes are highly regional in their prevalence.
Subtypes: FSIS will be conducting pulsed field gel electrophoresis (PFGE) on all salmonella on all positive carcasses in an effort to track infections back to specific plants. The problem is that PFGE subtypes are not fingerprints. They are highly conserved, and 40 percent of all salmonella have the same PFGE subtypes, regardless of whether they are found on chickens or tomatoes.
According to the FSIS report, the Agency is beginning to use the level of common salmonella serotypes of significance in terms of causing foodborne illnesses in evaluating performance of an establishment. For young chickens, 0-1 serotypes of human health significance in a verification set of 51 samples would be considered a low level, 2-4 would be a medium level, and 5 or more would be a high level. FSIS is now including the level of serotypes of human health significance in the End of Set letter it sends to each establishment upon completion of a verification sample set for salmonella. FSIS states that this serotype information will help an establishment in evaluating and improving its process control performance. This is a troubling statement. I am unaware of any research in the scientific literature that indicates that specific serotypes may be selected for or against during processing using interventions. In fact, there is no evidence that any plant has any control over the serotypes of salmonella that colonize the birds entering the plant..
Proposal 4: Levels of Inspection
FSIS proposes a Public Health Risk Based Inspection System (PHRBIS) that provides for automated responses to noncompliance reports (NRs) and establishes three different levels of inspection.
The USDA-FSIS indicates that it believes the poultry industry was not responding to NRs adequately. USDA stated that often, the plants would provide the explanation, "We will retrain our employees." At the meeting in Washington recently, the USDA mentioned that the Topps beef recall would not have happened had the company responded to the numerous NRs it had received. Therefore, the USDA is proposing to take the NR response by USDA inspectors out of their hands. All NRs will be entered into an online system that will automatically give the inspectors a response to carry out. Also, the online system will use an algorithm to calculate the risk of each type of NR and place the plant into a specific Level of Inspection (LOI). This takes the responsibility out of the hands of the inspectors. This system was designed to direct inspectors to "vulnerable points." To do this will require inspectors to be very highly trained in how to identify a vulnerable point and how to insure that it is controlled. The training associated with maintaining this level of expertise will be enormous.
Processors will be required to evaluate critical interventions in the process by conducting microbiological tests before and after each intervention to show the FSIS how well each of these "vulnerable points" are being controlled. Likewise, the plant will have to provide isolates of the salmonella and campylobacter found to the FSIS for further analysis. This requirement led the USPOULTRY to respond by stating, "Has FSIS considered the biosecurity implications of requiring poultry producers to ship samples that are known to contain salmonella and/or campylobacter? Has FSIS considered the additional cost incurred by the industry to secure the appropriate approvals/certifications that are required to be able to conduct such shipping activities?" These are valid concerns as one poultry company was recently fined $30,000 for shipping salmonella without having all of the required paperwork.
Levels of Inspection
LOI 1 means that inspection will go on as before. If a plant goes into an LOI 2, a Food Safety Assessment may be conducted in which a team from FSIS comes to the plant and conducts in-depth verification of procedures. This may increase the frequency of testing as well. Establishments in LOI 2 will be ranked based on their NRs by the algorithm to determine their potential public health impact. The problem is that the NR rankings of public health significance are specious at best. For example NRs concerning "a deviation of processing," violation of "general rules," "inadequate HACCP," and "zero tolerance for feces" are all weighted with a 3. Very few level 3-weighted NRs will cause an LOI 2 or 3. Thus, potentially, the plant could have one fecal failure, one piece of paper toweling from a mop go into the chiller, and 2 serotypes of salmonella linked to foodborne illness and it will be penalized. In reality, these things are not going to lead to foodborne illness.
When a plant reaches LOI 3, it will undergo an immediate Food Safety Assessment and increased salmonella testing by FSIS. The plant will be required to test all microbial interventions to determine their efficacy by evaluating salmonella, E. coli, and campylobacter. The Enforcement, Investigations and Analysis Officer (EIAO) will conduct in-depth verifications over a four- to six-week period. The plant will be allowed to remain in LOI 3 for only a short time (undefined) and then will be shut down. Under the new proposed baselines where a Category 2 will be >7.5 percent prevalence for salmonella, in combination with the ranking algorithm used for NRs, it has been estimated that greater than 90 percent of the plants would be penalized by this system.
Proposal 5: New FSIS baselines ahead
FSIS proposes updated baseline studies to better measure improvements in pathogen reduction in all classes of raw product. FSIS implemented the development of a baseline for young chickens in June 2007 and a young turkey baseline is scheduled for 2008.
Baselines are just a number and do not indicate the variables that significantly impact that baseline. For example, weather conditions in a given year, disease outbreaks and other factors will greatly impact salmonella prevalence, but are not evident when looking at a baseline value. Moreover, a baseline is an average. Saying that salmonella prevalence averaged 9.0 percent in 2007 does not give the person reading this information any context. If we have rampant outbreaks of disease, extremes in weather, we are going to have higher salmonella prevalence.
Far-reaching implications
A high percentage of the plants in the USA would be penalized under this system. Exports to large importing countries might well drop to zero. Companies would have to spend millions to comply and, in many cases, will not be able to solve their problems because they are not caused by a loss of process control. Plants will be ranked by consumers as low or high salmonella plants. Some will go out of business. The industry has been complaining for years that the FSIS does not apply the rules consistently, and I have observed this problem on numerous occasions myself. The rules are easy to apply now compared to the new ones that are going to be implemented. What will happen when these myriad regulations take effect?
Salmonella Initiative
Under the current regulation, following is how FSIS defines the three plant performance categories based on a sample set of 51 carcasses:
Category 1:
- Establishments with percent positive salmonella samples at 50 percent (six carcasses) or less of the performance standard or guideline in the two most recent completed sample sets.
Category 2:
- Establishments with percent positive salmonella samples above 50 percent but not exceeding the standard/guideline (seven to 12 carcasses) in the most recent completed sample set.
Category 3:
- Establishments with percent positive salmonella samples exceeding the standard/guideline (13 or more carcasses) in the most recent completed sample set.
Under the new proposed regulation, following is how the three plant performance categories would be defined based on a sample set of 51 carcasses:
Category 1:
- Establishments with percent positive salmonella samples at four carcasses or less of the performance standard or guideline in the two most recent completed sample sets.
Category 2:
- Establishments with percent positive salmonella samples above 50 percent but not exceeding the standard/guideline (five to 10 carcasses) in the most recent completed sample set.
Category 3:
- Establishments with percent positive salmonella samples exceeding the standard/guideline (11 or more carcasses) in the most recent completed sample set.